 Review Article
Review Article
A Review on The Role of Breeding for Stem Rust Disease Resistance in Wheat (Triticum Aestivum L.)
Birkneh Kuru Dosegnaw*
Ethiopian Institute of Agricultural Research, Pawe Agricultural Research Center, Ethopia
Birkneh Kuru Dosegnaw, Ethiopian Institute of Agricultural Research, Pawe Agricultural Research Center, Ethopia.
Received Date:June 07, 2022; Published Date: June 17, 2022
Abstract
Wheat (Triticum aestivum L.) is a key grain crop that serves for foods worldwide. It involves in developing countries is rising in recent decades and is predicted to realize 60% through 2050. However, the present erupt of a latest wheat stem rust race proficient of parasitizing many commercial wheat cultivars shows the superior breeding flow closer to the utilization of quantitative disease resistance genes and resistance gene pyramids have gotten accustomed warfare wheat stem rust and different diseases. The objective of this review is to brief the role of breeding to stem rust resistance wheat variety development everywhere the globe. The advancement in thoughtfulness on the molecular foundation of disease resistance at both host and non-host planes put forwards for further possibilities of stem rust resistance variety development by using biotechnological approaches. Nearly 58 stem rust resistance (Sr) genes are identified. together with these genes, a minimum of 27 genes, including Sr27, Sr28, Sr32, Sr33, Sr35, Sr36, Sr37, Sr39, Sr40, Sr42, Sr44, Sr45, Sr46, Sr47, Sr51, Sr52, Sr53, Sr55 (Lr67/Yr46/ Pm46) and (Lr46/Yr29/Pm39), are effectual or partially effective against the Ug99 race group.
Keywords:Ug99; Resistance gene; Gene pyramiding; Avirulence; Gene silencing; Combating stem rust; Sr-gene
Introduction
Wheat (Triticum aestivum L.) is a main food grain source worldwide. Its demand in developing countries is rising in recent decades and is predicted to achieve 60% by 2050 [1]. Wheat consumption in Africa has increased significantly since, the mid 1990’s, faster than the other major foodstuff. This has resulted in a very growing reliance on wheat imports, as wheat production in Africa has didn’t carry on with demand [2]. Wheat is a very important staple food within the diets of several Ethiopian, providing about 15% of the caloric share for the country’s over 90 million populations, set it 2nd following maize and somewhat preceding to teff, sorghum, and enset, which put in 10-12 percent each [3]. Diseases, insects and weeds are major obstacles in crop production throughout the globe. Under epidemic conditions they’ll cause complete loss of the crop. One of the most significant diseases of wheat is stem rust [4]. Strategic introgression and the use of resistance genes in commercial cultivars in the 1950s largely avoided a black rust pandemic. However, in additional recent years, the evolution and selection for brand new races with increased virulence became undesirable frequent reason behind yield loss [5]. The rust pathogens are a big and major constraint to wheat production across Africa. Wheat-growing areas in East Africa have proven to be hotspots for the emergence of the latest race of wheat rust, especially stem and yellow rust [6]. Puccinia gramminis is the cause of black rust and is therefore the most devastating disease with possible complete crop loss under favorable conditions [7].
The re-emergence of new set of races of Ug99 family” of stem rust in the last decade has been the most threats of wheat production. The first identifiable variant of this family is the first characterized race of Ug99, also known as TTKSK, based on its effect on the differences in selected host resistance genes [4]. His race has unique virulence to Sr31 and Sr38 resistance genes widely utilized in wheat worldwide and for which virulence had not been reported previously in the world [8]. Up to 70% yield losses are resulted due to the foundation of Ug99 and about 80% to 90% of the wheat grown is susceptible to stem rust globally [6]. Yield losses of 100% have been reported in Kenya, related with stem rust (Niau, et al. 2010). Only a small number of older varieties present a few levels of ‘adult plant resistance’ (APR) (Niau, et al. 2010) and [9]. while 95% of the local and commercial varieties are vulnerable or highly susceptible to disease. Chemicals can also be used to control black stem rust, but the main challenge is high cost and residual effects have a negative impact on the environment [10]. Therefore, genetic resistance is mainly cost-effective and feasible way to combat black stem rust.
Around seventy genes are selected for resistance up so far [11]. However, from these around thirty four genes are in ineffective against race Ug99 [12]. the worldwide look for new genes or gene combinations was championed by Dr. Norman Borlaug which brings the concept of “breeding for durable resistance in wheat” which intern accompany best opportunity to combat the new stem rust race Ug99 that would be released as new varieties. According to [13] a decent source of resistance to stem rust that would be deployed within the national wheat improvement program is that exotic wheat genotypes. Ug99 family of stem rust races features a devastating nature for wheat productivity regionally besides to those the trouble to seem at for resistance foundation and incorporation of efficient genes into new high yield commercial varieties is paramount.
In recent time the research work has been focused mainly on
genetic improvement for grain yield of wheat than breeding for
disease resistance. Breeding programs will not have much work to
do to obtain resistance in the genotypes adapted to the region, even
if resistance has been identified. This makes gradual reducing on
farm potential of different wheat variety because of the different
species of rust. This review is conducted to investigate the scope
of genetic variability of available genetic resources and explore
the potential for improvement through breeding programs and
strategic introgression and exploitation of resistance genes in
commercial varieties the greatly circumvented major stem rust
epidemics. Therefore, the objective of this review was:
1. To review the roles of breeding for stem rust disease resistance
in wheat.
2. To identify suitable sources of resistance gene for stem rust in
wheat.
Literature Review
Ideal conditions for wheat stem rust proliferation
An unusually cold spring followed by high early summer temperatures is best condition for spore germination rates, appressorium formation and penetration rates over the past quarter century and drove the model using microclimate estimates from the JRA-55 climate re-analysis [14]. Burrage SW [15] Put forward that the disease is most likely to occur in the summer; therefore, paying attention on weather data from June–August. Leaf wetness and optimal temperature range are the determinants of modeled spore germination and appressorium formation rates. Burrage SW [15] Suggested that the expected penetration rates are dependent on maximum temperature and light levels as well as moisture on leaf. Ideally stem rust growth requires the warmest temperature of the three rust types and the suite temperature level is that 59 to 84 °F and 6 to 8 hours of moisture on their leaf. In addition, new lesion is formed in seven to10 days with moist and optimum temperature.
Wheat stem rust symptoms
Puccinia graminis is a fungus which cause to stem, black and cereal rusts which puts significant effect on cereal crops. Stem rust infected plant produces fewer tillers, locate fewer seed it may result in die of the plant in cases of cruel infection. The fungus can cause yield losses of cereals in many ways this includes absorbing of nutrients that used for grain development or grain filling [16]. Shriveled grains and stems weaken, which can direct to lodging are the result of stem rust interference with plant vascular tissue. Stem weakness which leads to lodging and Shriveled grains are result of stem rust intervention within plant vascular tissue. Stem rust on wheat has many characteristics from thus the one is that the presence of uredinia which are brick-red, elongated, blister-like pustules that are easily shaken off. Uredinia develop on the leaves mostly on the underneath but might go through to the upper side. Pustules break the epidermis, giving a ragged appearance on leaf sheaths and glumes [4]. Stem rust is also known as black rust since it produced black telia when it reaches to the end of the growing season [17].
Defense mechanisms in wheat to steam rust
Application of chemical and genetic mechanisms is the two ways to manage rust in cereals. Genetic control is best over fungicides use for the reason that of the chance that the pathogens build up resistance to fungicides and furthermore for environmental and economic reasons [18]. There are two main classes of gene that helps to develop resistance, and which are used by wheat breeders based on their phenotypic effect which are strain specific (R) genes) and adult plant resistance (APR) genes. Race nonspecific genes most of the effect at adult growth stage while, race specific mainly effective from seedling to adult plant growth stage. There are no distinctions between strain specific and race nonspecific while, both classes are designed wheat stem rust resistance with increasing number to have room for newly discovered genes. Nowadays less focus is given for using of race specific resistance genes by some breeders and pathologists while, they put more focus on the discovery, characterization and use of race nonspecific genes for the development of long lasting resistance. Because, their re-assessment of the risk of rust fungi developing resistance to fungicides [18].
Role of breeding and strategies for wheat stem rust management
It is more applicable to sustainable agriculture, reduces the need for chemical applications and thus, environmental damage while serving as the best long-term solution for disease management [19]. Based on their economic importance for wheat production in Ethiopia, four predominant stem rust races; TTKSK, TKTTF, TRTTF and JRCQC were identified for seedling evaluation based on available data indicates. Three of them (TTKSK, TKTTF and TRTTF) are more important stem rust race for bread wheat species and race JRCQC is for durum wheat species [20] (Table 1).
Table 1:Description of single stem rust races used for seedling and adult plant evaluation.
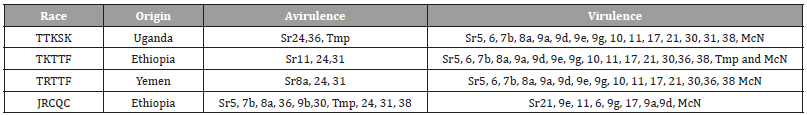
Source: [40]
The diploid wheat species Triticum monococcum genome is a well brought-up source of resistance genes. Chromosomes of hexaploid wheat rejoin defectively with the chromosomes of T. monococcum in the occurrence of the pairing homologous gene. Nevertheless, the homologous chromosomes from these two species rejoin more often than not in cause of once when this gene is deleted [21]. In this diploid species five loci have been postulated that they are presenting resistance to the complex Ug99 race [22]. Among them, Sr35 and Sr22, whereas Sr21 (Zhao et al., 2015) and SrTm4 [23] have been cloned and genetically mapped respectively. Triticum monococcum accession PI306540, which is obtained from Romania, is a basic starting place of multiple stem rust resistance genes. Cross between PI 306540 and stem rust-vulnerable accession PI 272557 produces a progeny that presents three genes efficient to strain TTKSK, two genes efficient to strain QFCSC, and one gene effective to strain TTTTF [22]. The gene Sr21 was expected to be efficient for one of the three strains which is TTKSK in PI 306540 however, it does not award resistance to the strain QFCSC or TTTTF [24]. SrTm4 is described as a second resistance gene in PI306540, which give resistance to strain TTKSK, TTTTF and QFCSC (Briggs et al., 2015). The third gene efficient to strain TTKSK in PI 306540 is referred as SrTm5.
RNA interference and host-induced gene silencing
Intraspecific genetic diversity will be impacted by global climate change in a variety of ways. Changes in the distribution of genetic variants in space and time as population and species ranges change, changes in the levels of phenotypic plasticity of individuals and populations as they respond to new environmental conditions, and evolutionary adaptation to changing environmental conditions are all examples of these [25]. These resulted in increasing reduction of genetic diversity which inter resulted in increasing of genetic vulnerability of crop species for different plant disease from both internal and external. As a result, different plant genetic modification is used to combat the issue of biotic and abiotic stress among those methods the one is gene silencing.
Gene silencing controls gene expression and metabolism all over the life of an organism through negative feedback mechanisms to define the fate of those organisms. Immunity to biological stress is provided by gene silencing [26]. By recognizing abnormal RNA through nonsense-mediated messenger RNA (mRNA) disruption (NMD), it plays an important role in the defense mechanism of plants. This can be fatal if left in the cell’s RNA pool. Gene silencing causes a regular shutdown Regular shutdown of gene expression at the mRNA or protein level caused by gene silencing. It also regulates the spatiotemporal of gene networks and then regulates the developmental processes of plant metabolism. B. Genome stability, detoxification of plant waste and allergens [27]. Silence of transgenes, such as the petunia-transformed CHSA gene, to dark brown adversely affects gene transformation, but albino phenotypes have been observed in transgenic plants [28].
In tobacco plants, the result of gene silencing on endogenous genes has also been observed, with the development of coinhibition of nitrite reductase, nitrate reductase, and SAM synthase resulting in vascular necrosis or chlorotic phenotype, gene silencing and genetic damage. It shows the relationship with [29]. Based on RNA silencing mechanisms, plants naturally develop an immune system against invading viruses [30]. [31] has been proposed that RNA silencing be used to develop host-induced gene silencing techniques for controlling other plant pathogens [31] HIGS allows for gene silencing of phytopathogens by expressing RNA interference (RNAi) constructed against specific endogenous genes of the host plant pathogen, extra growth virus-inducing gene silencing (VIGS).
The production of double-stranded RNA (dsRNA) in transgenic plants is used to form small RNAs, but for experimental purposes, dsRNAs are transferred to plant cells using agro bacterium or virus that replicates via dsRNAs. In plants, animals and fungi, RNA silencing can be introduced which is an exceedingly conserved mechanism for host-induced development of the immune system [32]. Transgenic modification technology based on the placement of reverse repeat sequences in the plant genome is a technology that is carried by host-induced gene silencing. Sequences homologous to pathogen-derived genes incorporated into the plant genome articulate genes that target siRNAs, causing pathogen gene silencing. Various host-induced gene silencing technology vectors have been developed to drive long dsRNA and hairpin sequences hooked on the plant genome via reverse repeat genes or reverse promoter sequences. Not only does RNAi in higher plants order gene expression in the body itself, but the placement of foreign dsRNA in plants has recently become a traditional form of crop protection. Transposons migrate within the genome and pose a serious threat to the stability of the genome. This is blocked by gene silencing [33].
Gene silencing maintains the balance of transcripts and ensures that plants adapt to environmental changes. Therefore, if a particular crop has greater genetic diversity, it will have greater adaptation to changing environmental conditions. Wheat, for example, adapts to different environments due to its bio-phenological genetic diversity which consist of vernalization (Vrn), photoperiod (Ppd), and dwarfism (Rht) genes. Alleles and facsimile number polymorphisms identified for the Vrn and Ppd genes reacted differently to different climatic conditions and can alter yield as well as developmental stages [69]. The system of flowering near to similar period of time to a wider range of climatic conditions is primarily regulated by the Vrn gene (exposure to cold demand), the Ppd gene (photoperiod sensitivity), and the autonomous earliness itself (Eps) genes [71].
Wide hybridization
At the middle of the 20century cytogeneticists, breeders and farmers carried extensive crosses between wheat and a number of grass species. Wheat wide hybridization breeding has a vast potential and worth long-lasting, specifically in the aspect of improving wheat wide adaptability to biotic and abiotic stresses. Extensive research was motivated by the desire to summarize the traits of many desirable exotic species of wheat. Though disease resistance was one of the most sought-after traits, additional characters have included increased yield [34], early maturity drought tolerance [35] lodging resistance [36], cold tolerance [Rakesh and Sethi,] and high protein content [37]. To get better of these characters the test material includes, or we focused on primary, secondary and tertiary which is contained in varied set of the Triticeae species. These gene pools are composed of wild and cultivated species of the genus Aegilops, Agropyron, Ambylopyrum, Dasypyrum, Elymus, Hordeum, Leymus, Lophopyrum, Psathyrostachys, Pseudoroegneria, Secrete, Thinopyrum and Triticum.
In other cause wide hybridization includes cross of plants out of their genera these can be carried through the system of ivitro culture and regeneration of plants is the prerequisite for modifying plants by means of genetic engineering. Different crops are hybridized or engineered through this system for a number of required traits to have from any sources for that particular gene or trait. Those with genes to improve the quality of the product, with genes to allow them to resist disease, insect pests, plants with genes that allow them to be resistant to the effects of herbicides, as well as plants with genes conferring resistance to environmental conditions that cause crop losses because of extremes of cold, heat, drought, salt concentration [38]. Example in rice the trans-gene Cry1A (b) & Cry1A (c) is taken from bacteria and used Generate plants resistant to yellow stem borer [39]. A trans-gene, called the osmotin gene, inserted into rapeseed from mustard or tobacco produces herbicide- tolerant plants, male sterility and recovery lines for hybrid seed production [40].
Resistance genes
Cultivars own genes which are powerful within the specific geographic area in which the cultivars are maximum likely to be grown [25]. Contemporary research on stem rust has focused on identifying extra resistance genes to manipulate Ug99. Approximately fifty-eight resistance (Sr) genes were recognized [41] and [42] and numerically specified as Sr1 to Sr58 for stem rust as part of the worldwide Wheat Genetics Symposium Gene Catalog. Together with these genes, at least 27 genes, which includes Sr2 (Yr30), Sr13, Sr21, Sr22, Sr24, Sr25, Sr26, Sr27, Sr28, Sr32, Sr33, Sr35, Sr36, Sr37, Sr39, Sr40, Sr42, Sr44, Sr45, Sr46, Sr47, Sr51, Sr52, Sr53 and Sr55 (Lr67/Yr46/ Pm46) and (Lr46/Yr29/Pm39) are efficient or partly green towards the Ug99 race group [43,44,45,46] amongst those 27 genes, Sr2, Sr13, Sr22, Sr25, Sr26, Sr35, Sr39 and Sr40 have been stated to be the only against Ug99 [46].
Understanding the genetic basis for durable resistance to stem rust sickness is important for improving the efficiency of wheat breeding [47,48] reported that one of the extra effective stem rust resistance genes Sr2 is a catalogued race nonspecific gene in wheat and has been proven the use of SSR markers to be positioned in an area on the short arm of chromosome 3B. That is derived from the variety ‘hope and normally called the ‘Sr2- complicated’ (Sr2+Yr30+Lr27+Pbc) [49]. Because of its capacity to bestow durable and broad-spectrum resistance, the Sr2 gene has been used to get better resistance against stem rust diseases in wheat varieties cultivated worldwide [37]; [46] and [50]. Sr35 was a resistance gene initially relocated from Triticum monococcum to hexaploid wheat (Neu et al., 2012). Also, it is efficient against the TTKSK (Ug99) and it’s variant [51].
Global distribution of wheat cultivars containing the Sr gene:Around 60% of the present CIMMYT spring wheat germplasms comprise Sr2 genes (Singh et al., 2008). Likewise, greater than fifty percent of the (ICARDA) and South African wheat germplasms have proven SSR haplotype courting with the long-lasting black stem rust resistance Sr2 gene (Ogbonnoya and Francis, 2011). Grouping of Sr2 with the uncharacterized gradual-rusting genes typically referred to as the Sr2-complicated has furnished the inspiration for long-lasting resistance to stem rust in maximum components of the world [52]. Sr24 gene is a stem rust resistance that effectively conferred resistance towards most races of Pgt and is found in most commercial wheat cultivars globally [53] and [54]. Greater than 20% of the South African wheat elite lines and germplasms convey Sr24 as a first-rate resistance gene. The stem rust resistance genes Sr25 and Sr26 are effective towards variants of Ug99, TTKST and TTTSK [6] and [55].
Resistance (R) gene pyramiding
Gene pyramiding involves stacking several preferable and attractive genes into a particular genotype has been suggested as one way to surmount resistance instability conferred by using single gene resistance in many pathogens such as soybean rust [56] and [57]. Gene pyramiding or stacking calls for breeders to combine several parents in order to develop elite lines and improved varieties [58].
Race-specific resistance:Race-specific resistance or Seedling resistance, typically conferred by a single gene or simple combinations of single genes, is a type of resistance that can be perceived at the seedling stage and remains efficient throughout all stages of plant growth [68] and [59] .Genes which are powerful in opposition to a few however now no longer all races of a rust pathogen and generally be traditional to the classical gene-forgene version are known as Race- unique resistance genes, wherein resistance relies upon on a selected genetic interplay among hostresistance (R) genes and pathogen avirulence (Avr) genes. R genes in plant life predominantly encode nucleotide-binding and leucinewealthy repeat (NLR) proteins, which act as immune receptors to comprehend pathogen effector proteins introduced into host cells for the duration of infection [60]. A flax rust pathology system develops nineteen NLR-encoding R genes and six corresponding Avr gene families which used for rust resistance mechanisms investigation (Anderson et al., 2019 and Ravensadale et al., 2011). As a result, strain-specific resistance genes have become more attractive to breeding programs and have provided significant economic benefits to wheat disease control. Despite these advantages, the resistance conferred by the seedling resistance gene is often short-lived because it cannot provide an economical level of protection for prolonged time [61]. The lack of permanence of species-specific resistance is primarily due to susceptibility to the rapid development of the corresponding pathogen in the pathogen and can overcome the efficacy of such resistance genes [62]. Many cultivar-specific resistance genes have been genetically characterized in wheat and various crops, and more and more clones are being made today. Nowadays the development of NLR gene capture tactics is used to assure to rapidly expand the variety of cloned rust-resistance genes [62]. Stem rust resistance genes (Sr22, Sr33, Sr35, Sr45, and Sr50), pathogens were cloned, and all encode NLR receptor proteins [62] and [63]. R-gene capture strategies can be used to locate and discover NLR genes, with the aim of those NLRs pyramided into R-gene to offer long lasting resistance.
Adult plant or race non-specific resistance:Race non-specific resistance is a host defense mechanism that confers quantitative and partial protection against the invading pathogen in a race nonspecific manner [64]. Seedlings of wheat plants carrying adult plant resistance are susceptible to the disease but become effective and provide varying planes of resistance to stem rust at various stages of post-seedling crop growth [59]. Adult plant resistance is often conditioned by quantitative trait loci that might have small individual effects in reducing disease development but operate jointly in an additive approach to grant a high level of resistance. Adult plant resistance is often characterized by its effectiveness in anticipation of a broad range of pathogen races and has been considered more durable, providing resistance without being readily overcome by the change in pathogen virulence when the cultivar is widely grown in an area where the disease is prevalent [65].
The deployment of cultivars carrying APR based on multiple genes is particularly preferred to delay infection, growth and reproduction of the pathogen in adult plants and circumvent “boom-and-bust” cycles [64] and [66]. Despite the advantages of the quantitatively inherited APR genes in resistance, and the fact that such genes are common within the primary gene pool and further, only limited number of apparent resistance genes has been deliberately deployed by breeding programs. The complex inheritance of highly effective APR resistance presents a relative difficulty in identification and routine deployment of this type resistance in wheat breeding [67]. In wheat, APR is often manifested only at later stages of development that is why it to be called adult plant resistance (APR). In contrast to the R gene, which encodes most NLRs, in a number of APR genes have proven to be very longlived. Near hundred years Sr2 has been efficient on the ground alongside various types of black rust [68]. Nowadays cloning of different wheat APR genes provided imminent into the mechanism of non-breed-specific resistance.
Conclusion
Wheat Black stem rust is one of the most devastating wheat diseases that causes significant yield reductions in wheat growing areas around the world which is caused by Pucciniagraminisf. Sp. Tritici [69,70]. The best mechanisms of controlling wheat stem rust are that using of disease resistant cultivars. The four predominant wheat stem rust races are TTKSK, TKTTF, TRTTF and JRCQC. To control steam rust, host genetic resistance remains the most economically viable, environmentally friendly and strategically essential alternative for aid-confined farmers in the growing world. Race-particular resistance genes are effective towards some however not all races of a rust pathogen and commonly comply with the classical gene-for-gene version, where resistance relies upon on a selected genetic interaction among host-resistance (R) genes and pathogen virulence (Avr) genes [71,72]. Race-particular genes typically provide highly powerful resistance and are incredibly clean to incorporate into commercial cultivars because of easy inheritance. As an end result, race specific resistance genes were extra appealing to breeding applications and feature furnished enormous financial advantages in controlling rust ailment in wheat [73,74]. Despite these advantages, but resistances conferred with the aid of seedling resistance genes are regularly short lived, lacking the ability to offer a financial degree of safety over a prolonged time frame. The use of adult plant resistance mechanism is more powerful than race particular resistance. since the excessive variability and difficulty in whole resistance of wheat stem rust, typically plant breeding has crucial role in wheat stem rust resistant that’s a very extreme chance to wheat production globally for the reason that it’s miles environmental sound and economically feasible for the ones farmers that haven’t any ability to prevent the pathogen via chemical manipulate [75-78].
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Akram S, Arif MAR, Hameed A (2021) A GBS-baseds GWAS analysis of adaptability and yield traits in bread wheat (Triticum aestivum L.). Journal of Applied Genetics 62(1): 27-41.
- Mason NM, Jayne TS & Shiferaw BA (2012) Wheat consumption in Sub-Saharan Africa: trends, drivers, and policy implications (No. 1096-2016-88381).
- Minot N, Warner J, Lemma S, Kasa L, Gashaw A, et al. (2019) The wheat supply chain in Ethiopia: Patterns, trends, and policy options. Gates Open Res 3(174): 174.
- Singh RP, Hodson DP, Huerta Espino J, Jin Y, Njau P, et al. (2008) Will stem rust destroy the world's wheat crop. Advances in agronomy 98: 271-309.
- Singh A, Knox RE, DePauw RM, Singh AK, Cuthbert RD, et al. (2013) Identification and mapping in spring wheat of genetic factors controlling stem rust resistance and the study of their epistatic interactions across multiple environments. Theoretical and Applied Genetics 126(8): 1951-1964.
- Singh RP, Hodson DP, JinY, Huerta Espino J, Kinyua MG, et al. (2006) Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB reviews: perspectives in agriculture, veterinary science, nutrition and natural resources 1(54): 1-13.
- Pretorius ZA, Singh RP, Wagoire WW, Payne TS (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis. f. sp. tritici in Uganda. Plant Disease 84(2): 203-203.
- Vergara Diaz O, Kefauver SC, Elazab A, Nieto Taladriz MT, Araus JL (2015) Grain yield losses in yellow-rusted durum wheat estimated using digital and conventional parameters under field conditions. The Crop Journal 3(3): 200-210.
- Wanyera R, Macharia JK, Kilonzo SM, Kamundia JW (2009) Foliar fungicides to control wheat stem rust, race TTKS (Ug99), in Kenya. Plant disease 93(9): 929-932.
- Beard C, Jayasena K, Thomas G, Loughman R (2006) Managing stem rust of wheat. Managing stem rust of wheat v. 73.
- McIntosh RA, Dubcovsky J, Rogers JW, Morris CF, Appels R, et al. (2014) Catalogue of gene symbols for wheat: 2013-14 Supplement. Annual wheat newsletter p. 58.
- Singh RP, Hodson DP, Jin Y, Lagudah ES, Ayliffe MA, et al. (2015) Emergence and spread of new races of wheat stem rust fungus: continued threat to food security and prospects of genetic control. Phytopathology 105(7): 872-884.
- Watson A (2018) Speed breeding with genomic selection to accelerate genetic gain for yield in spring wheat (Triticum aestivum).
- Kobayashi S, Ota Y, Harada Y, Ebita A, Moriya M, et al. (2015) The JRA-55 reanalysis: general specifications and basic characteristics. Journal of the Meteorological Society of Japan. Ser II 93(1): 5-48.
- Burrage SW (2009) Environmental factors influencing the infection of wheat by Puccinia graminis. Annals of Applied Biology 66(3): 429-440.
- Schumann GL & Leonard KJ (2011) Stem rust of wheat.
- CIMMYT M (2005) Sounding the alarm on Global Stem rust: An assessment of Race Ug99 in Kenya and Ethiopia and the potential for impact in Nighboring countries and beyond. CIMMYT, Mexico City, Mexico.
- Oliver RP (2014) A reassessment of the risk of rust fungi developing resistance to fungicides. Pest management science 70(11): 1641-1645.
- Huerta Espino J, Roelfs AP (1992) Leaf rust on durum wheats. Vortraege fuer Pflanzenzuechtung (Germany).
- Hailu E, Woldaeb G, Danbali W, Alemu W, Abebe T (2015) Distribution of Stem Rust (Puccinia graminis f. sp. tritici) Races in Ethiopia. Advances in Crop Science and Technology pp. 1-4.
- Singh RP, Ma H & Rajaram S (1995) Genetic analysis of resistance to scab in spring wheat cultivar Frontana. Plant Disease 79(3): 238-240.
- Rouse MN, Olson E L, Gill BS, Pumphrey MO, Jin Y (2011) Stem rust resistance in Aegilops tauschii germplasm. Crop Science 51(5): 2074-2078.
- Briggs, Jordan, Shisheng Chen, Wenjun Zhang, Sarah Nelson, et al. (2015) "Mapping of SrTm4, a recessive stem rust resistance gene from diploid wheat effective to Ug99." Phytopathology 105 no. 10: 1347-1354.
- Zhao J, Zhao S, Chen X, Wang Z, Wang L, et al. (2015) Determination of the role of Berberis spp. in wheat stem rust in China. Plant disease 99(8): 1113-1117.
- Pauls SU, Nowak C, Bálint M, Pfenninger M (2013) The impact of global climate change on genetic diversity within populations and species. Molecular ecology 22(4): 925-946.
- El-Sappah AH, Yan K, Huang Q, Islam M, Li Q, et al. (2021) Comprehensive Mechanism of Gene Silencing and Its Role in Plant Growth and Development. Frontiers in Plant Science 1891.
- Mirouze M, Reinders J, Bucher E, Nishimura T, Schneeberger K, et al. (2009) Selective epigenetic control of retrotransposition in Arabidopsis. Nature 461(7262): 427-430.
- Napoli C, Lemieux C, Jorgensen R (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. The plant cell 2(4): 279-289.
- Palauqui JC, Elmayan T, de Borne FD, Crété P, Charles C, et al. (1996) Frequencies, timing, and spatial patterns of co-suppression of nitrate reductase and nitrite reductase in transgenic tobacco plants. Plant Physiology 112(4): 1447-1456.
- Csorba T, Pantaleo V, Burgyán J (2009) RNA silencing: an antiviral mechanism. Advances in virus research 75: 235-230.
- Harvey JJ, Lewsey MG, Patel K, Westwood J, Heimstädt S, et al. (2011) An antiviral defense role of AGO2 in plants. PloS one 6(1): 14639.
- Jorgensen R (2006) Plants, RNAi, and the Nobel prize. Science 314(5803): 1242-1243.
- Slotkin RK, Martienssen R (2007) Transposable elements and the epigenetic regulation of the genome. Nature reviews genetics 8(4): 272-285.
- Reynolds MP, Calderini DF, Condon AG, Rajaram S (2001) Physiological basis of yield gains in wheat associated with the LR19 translocation from Agropyron elongatum. In Wheat in a global environment pp. 345-351.
- Molnár Láng M, Molnár I, Szakács É, Linc G, Bedö Z (2014) Production and molecular cytogenetic identification of wheat-alien hybrids and introgression lines. In Genomics of plant genetic resources pp. 255-283.
- Chen X, Penman L, Wan A, Cheng P (2010) Virulence races of Puccinia striiformis f. sp. tritici in 2006 and 2007 and development of wheat stripe rust and distributions, dynamics, and evolutionary relationships of races from 2000 to 2007 in the United States. Canadian Journal of Plant Pathology 32(3): 315-333.
- De Pace C, Vaccino P, Caceres ME, Corbellini M (2011) Development of valuable wheat inbred lines through the introduction of Dasypyrum villosum germplasm in their pedigree.
- Anderson C, Khan MA, Catanzariti AM, Jack CA, Nemri A, et al. (2016) Genome analysis and avirulence gene cloning using a high-density RADseq linkage map of the flax rust fungus, Melampsora lini. BMC genomics 17(1): 1-20.
- Ramesh S, Nagadhara D, Reddy VD, Rao KV (2004) Production of transgenic indica rice resistant to yellow stem borer and sap-sucking insects, using super-binary vectors of Agrobacterium tumefaciens. Plant Science 166(4):1077-1085.
- Bansal KC, & Saha D (2013) Genetic Transformation and Crop Improvement. In Biotechnology in Agriculture and Food Processing pp. 149-184.
- Yu LX, Barbier H, Rouse MN, Singh S, Singh RP, et al. (2014) A consensus map for Ug99 stem rust resistance loci in wheat. Theoretical and Applied Genetics 127(7): 1561-1581.
- Haile JK, Hammer K, Badebo A, Singh RP, Röder MS (2013) Haplotype analysis of molecular markers linked to stem rust resistance genes in Ethiopian improved durum wheat varieties and tetraploid wheat landraces. Genetic resources and crop evolution 60(3): 853-864.
- Rouse MN, Jin Y (2011) Stem rust resistance in A-genome diploid relatives of wheat. Plant Disease 95(8): 941-944.
- Faris JD, Xu SS, Cai X, Friesen T L, Jin Y (2008) Molecular and cytogenetic characterization of a durum wheat–Aegilops speltoides chromosome translocation conferring resistance to stem rust. Chromosome Research 16(8): 1097-1105.
- Ghazvini H, Hiebert CW, Zegeye T, Liu S, Dilawari M, et al. (2012) Inheritance of resistance to Ug99 stem rust in wheat cultivar Norin 40 and genetic mapping of Sr42. Theoretical and Applied Genetics 125(4): 817-824.
- Baranova O A, Lapochkina IF, Anisimova AV, Gajnullin N R, Iordanskaya I V, et al. (2016) Identification of Sr genes in new common wheat sources of resistance to stem rust race Ug99 using molecular markers. Russian Journal of Genetics: Applied Research 6(3): 344-350.
- Yu LX, Lorenz A, Rutkoski J, Singh RP, Bhavani S, et al. (2011) Association mapping and gene–gene interaction for stem rust resistance in CIMMYT spring wheat germplasm. Theoretical and applied genetics 123(8): 1257-1268.
- Hare RA, McIntosh RA (1979) Genetic and cytogenetic studies of durable adult-plant resistances in" Hope" and related cultivars to wheat rusts.
- Singh RP, Huerta Espino J, Bhavani S, Singh D, Singh PK, et al. (2009) Breeding for minor gene-based adult plant resistance to stem rust in wheat. In Proceedings, oral papers and posters, 2009 Technical Workshop, Borlaug Global Rust Initiative, Cd. Obregón, Sonora, Mexico, pp. 131-139.
- Helguera M, Khan IA, Dubcovsky J (2000) Development of PCR markers for the wheat leaf rust resistance gene Lr47. Theoretical and Applied Genetics 100(7): 1137-1143.
- Rajaram S, Singh RP, Torres E (1988) Current CIMMYT approaches in breeding wheat for rust resistance. Chapter 9. In Breeding Strategies for Resistance to the Rusts of Wheat. El Batan, Mexico.
- Ejaz M, Iqbal M, Shahzad A, Ahmed I, Ali GM (2012) Genetic variation for markers linked to stem rust resistance genes in Pakistani wheat varieties. Crop Science 52(6): 2638-2648.
- Jin Y, Singh RP, Ward RW, Wanyera RUTH, Kinyua MIRIAM, et al. (2007) Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f. sp. tritici. Plant Disease 91(9): 1096-1099.
- Garcia A, Calvo ÉS, de Souza Kiihl RA, Harada A, Hiromoto DM, et al. (2008) Molecular mapping of soybean rust (Phakopsora pachyrhizi) resistance genes: discovery of a novel locus and alleles. Theoretical and Applied Genetics 117(4): 545-553.
- Lemos NG, de Lucca e Braccini A, Abdelnoor RV, de Oliveira MCN, Suenaga K, et al. (2011) Characterization of genes Rpp2, Rpp4, and Rpp5 for resistance to soybean rust. Euphytica 182(1): 53-64.
- Francis DM, Merk HL & Namuth-Covert D (2012) Gene pyramiding using molecular markers. Plant Breed. Genomics.
- Chen S, Rotaru AE, Liu F, Philips J, Woodard TL, et al. (2014) Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresource technology 173: 82-86.
- Jones DA (2016) Genome analysis and avirulence gene cloning using a high-density RADseq linkage map of the flax rust fungus, Melampsora lini. BMC genomics 17(1): 1-20.
- Johnson R (1984) A critical analysis of durable resistance. Annual review of phytopathology 22(1): 309-330.
- Steuernagel B, Periyannan SK, Hernández Pinzón I, Witek K, Rouse MN, et al. (2016) Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nature biotechnology 34(6): 652-655.
- Krattinger SG, Keller B (2016) Molecular genetics and evolution of disease resistance in cereals. New phytologist 212(2): 320-332.
- Chen XM (2005) Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Canadian journal of plant pathology 27(3): 314-337.
- McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers J Morris C, et al. (2013) Catalogue of gene symbols for wheat. In Proceedings of the 12th International Wheat Genetics Symposium pp. 8-13.
- Singh RP, Huerta Espino Julio, William HM (2005) Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. Turkish journal of agriculture and forestry 29(2): 121-127.
- Chen Y, Li H, Conner RL, Liu Z, Li Y, et al. (2007) Characterization of wheat-triticale lines resistant to powdery mildew, stem rust, stripe rust, wheat curl mite, and limitation on spread of WSMV. Plant disease 91(4): 368-374.
- Ellis JG, Lagudah ES, Spielmeyer W, & Dodds PN (2014) The past, present and future of breeding rust resistant wheat. Frontiers in plant science 5: 641.
- Ellis JG, Lagudah ES, Spielmeyer W, & Dodds PN (2014) The past, present and future of breeding rust resistant wheat. Frontiers in plant science 5: 641.
- Boyd LA (2005) Can Robigus defeat an old enemy? –Yellow rust of wheat. The Journal of Agricultural Science 143(4): 233-243.
- Dowla MNU, Edwards I, Ohara G, Islam S, Ma W (2018) Developing wheat for improved yield and adaptation under a changing climate: optimization of a few key genes. Engineering 4(4): 514-522.
- Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, et al. (2008) Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Disease 92(6): 923-926.
- Kato K, Yamagata H (1988) Method for evaluation of chilling requirement and narrow-sense earliness of wheat cultivars. Japanese Journal of Breeding 38(2): 172-186.
- Letta T (2018) Seedling Resistance to Stem Rust (Puccinia graminis f. sp. tritici) and Molecular Marker Analysis of Resistance Genes in Some Wheat Cultivars. Plant 6(1): 16-23.
- Neu C, Stein N, Keller B (2002) Genetic mapping of the Lr20 Pm1 resistance locus reveals suppressed recombination on chromosome arm 7AL in hexaploid wheat. Genome 45(4): 737-744.
- Njau PN, Jin Y, Huerta Espino J, Keller B, Singh RP (2010) Identification and evaluation of sources of resistance to stem rust race Ug99 in wheat. Plant Disease 94(4): 413-419.
- Ogbonnaya F (2010) 61-1 Characterization of Stem Rust Resistance in ICARDA/CWANA Elite Wheat Germplasm Using Linked Molecular Markers.
- Pauls SU, Nowak C, Bálint M, Pfenninger M (2013) The impact of global climate change on genetic diversity within populations and species. Molecular ecology 22(4): 925-946.
- Rakesh V, Sethi GS (2000) Cytogenetic analysis and differential response of rye-introgressed bread wheat genotypes for cold tolerance. Indian Journal of Genetics & Plant Breeding 60(1): 1-4.
- Ravensdale M, Nemri A, Thrall PH, Ellis JG, Dodds PN (2011) Co‐evolutionary interactions between host resistance and pathogen effector genes in flax rust disease. Molecular plant pathology 12(1): 93-102.
-
Birkneh Kuru Dosegnaw. A Review on The Role of Breeding for Stem Rust Disease Resistance in Wheat (Triticum Aestivum L.). World J Agri & Soil Sci. 8(1): 2022. WJASS.MS.ID.000679.
-
Ug99, Resistance gene, Gene pyramiding, Avirulence, Gene silencing, Combating stem rust, Sr-gene
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






